FEATURED POST
What You Can Learn from 7 Theme Fusion Success Stories
Nam lacinia arcu tortor, nec luctus nibh dignissim eu. Nulla sit amet maximus nulla. Pellentesque a accumsan eros, ac molestie nulla. Morbi interdum in neque vitae vulputate.
OMICS Technologies in Personalized Medicine on EJP RD’s MOOC
In a significant contribution to the European Joint Programme on Rare Diseases (EJP RD) “Innovative Therapies and Personalized Medicine for Rare Disease” [...]
Big Data and Machine Learning in Personalized Medicine: Insights from the EJP RD’s MOOC
The landscape of healthcare is rapidly evolving, with big data and machine learning at the forefront of this transformation. In the [...]
Donato Bonifazi, CVBF’s CEO to Represent TEDDY, the European Network of Excellence for Paediatric Research, in the ACT EU Multi-Stakeholder Platform Annual Meeting
We are pleased to announce that our CEO, Donato Bonifazi, a permanent member of the Advisory Group of the ACT EU Multistakeholder [...]
OrphaDev4Kids Project Commences with Kick-Off in Bari
The OrphaDev4Kids project, focused on advancing medical devices development for rare diseases in children, will officially launch with a kick-off [...]
EPTRI’s 2024 Scientific Meeting
The European Paediatric Translational Research Infrastructure (EPTRI) has exciting news to share about its upcoming Scientific Meeting and legal [...]
iCAN 2024 Advocacy and Research Summit
Mark your calendars! The 2024 iCAN Advocacy and Research Summit is scheduled to take place from 15-19 July in Bari, [...]
CVBF’s Researchers Publications in Paediatric Data Standardization
Recent publications from researchers affiliated with the CVBF consortium underscore our commitment to advancing efforts in paediatric data standardization. The [...]
CVBF’s CEO selected as Permanent Member of the Advisory Group of the ACT EU Multistakeholder Platform (MSP AG)
On 20 December 2023, Donato Bonifazi, our CEO, has been selected as a permanent member of the Advisory Group of the ACT EU Multistakeholder [...]
CVBF Wins ERAMET Project, Funded by Horizon Europe
CVBF has achieved a major milestone by securing a significant role in the Horizon Europe-funded ERAMET project. Guided by Prof Adriana Ceci, the Lead Scientist [...]
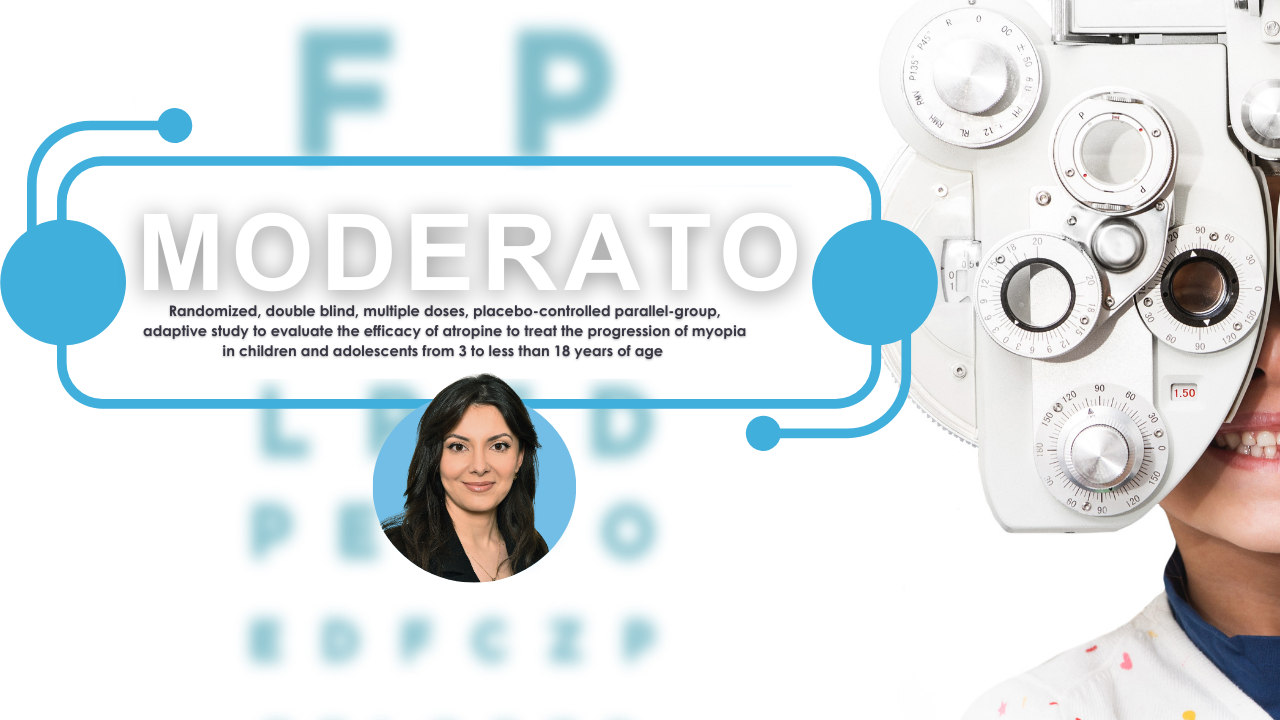
New Clinical Trial Seeks to Slow Progression of Nearsightedness in Children
A new international clinical trial is underway across Europe to test whether low-dose atropine eye drops can effectively slow the progression of myopia (nearsightedness) in [...]
13 Organizations Across Europe Join Forces for Novel ATMP on Faecal incontinence.
CVBF provides regulatory, site management, and monitoring support for the AMELIE (Anchored Muscle cELls for IncontinencE) project, Ana Dilo as our Clinical Project Manager of [...]
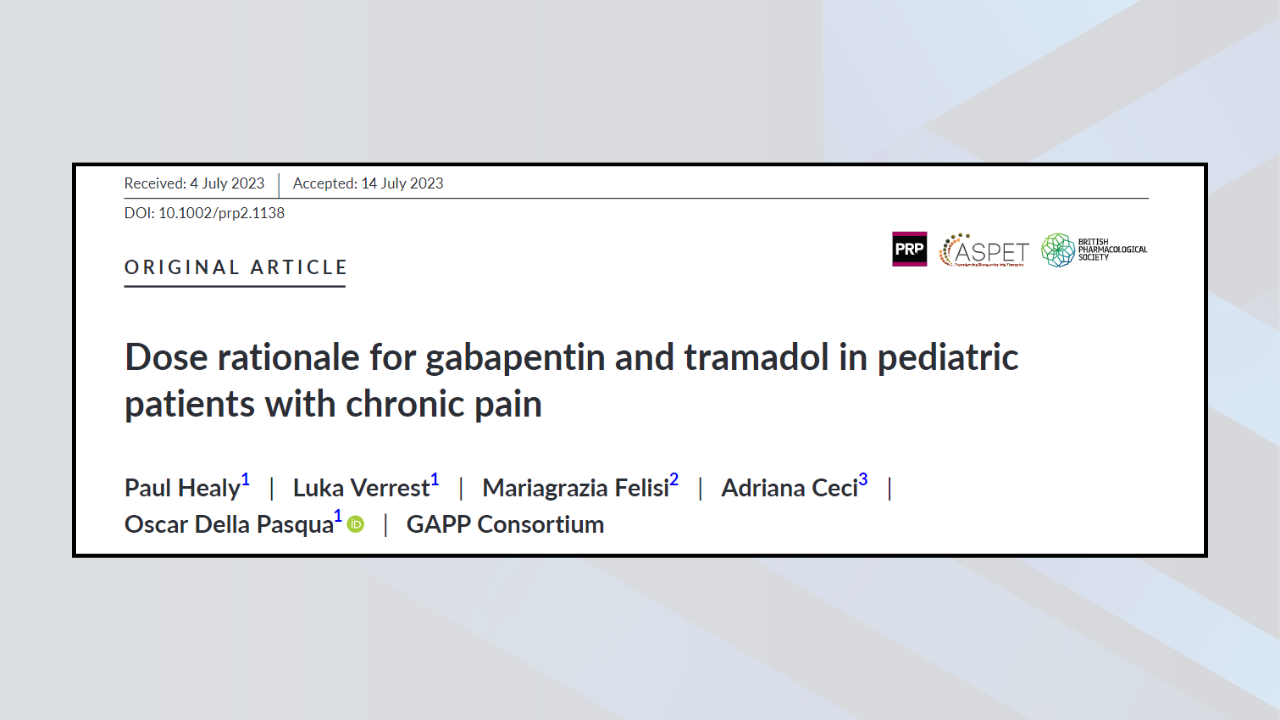
New Article on Gabapentin and Tramadol in Paediatric Patients with Chronic Pain
A new article published on 6 October 2023 in the Journal of the British Pharmacological Society titled"Dose rationale for gabapentin and tramadol in paediatric patients [...]
Quality Assurance Head, Mariagrazia Felisi, Leads Workshop on EU Clinical Trial Regulation
On 27 June 2023, Mariagrazia Felisi, our Quality Assurance Head and Internal Auditor, co-chairing the "AICRO Clinical Trial Center Working Group", opened and led the [...]