FEATURED POST
What You Can Learn from 7 Theme Fusion Success Stories
Nam lacinia arcu tortor, nec luctus nibh dignissim eu. Nulla sit amet maximus nulla. Pellentesque a accumsan eros, ac molestie nulla. Morbi interdum in neque vitae vulputate.
CVBF Wins ERAMET Project, Funded by Horizon Europe
CVBF has achieved a major milestone by securing a significant role in the Horizon Europe-funded ERAMET project. Guided by Prof Adriana Ceci, [...]
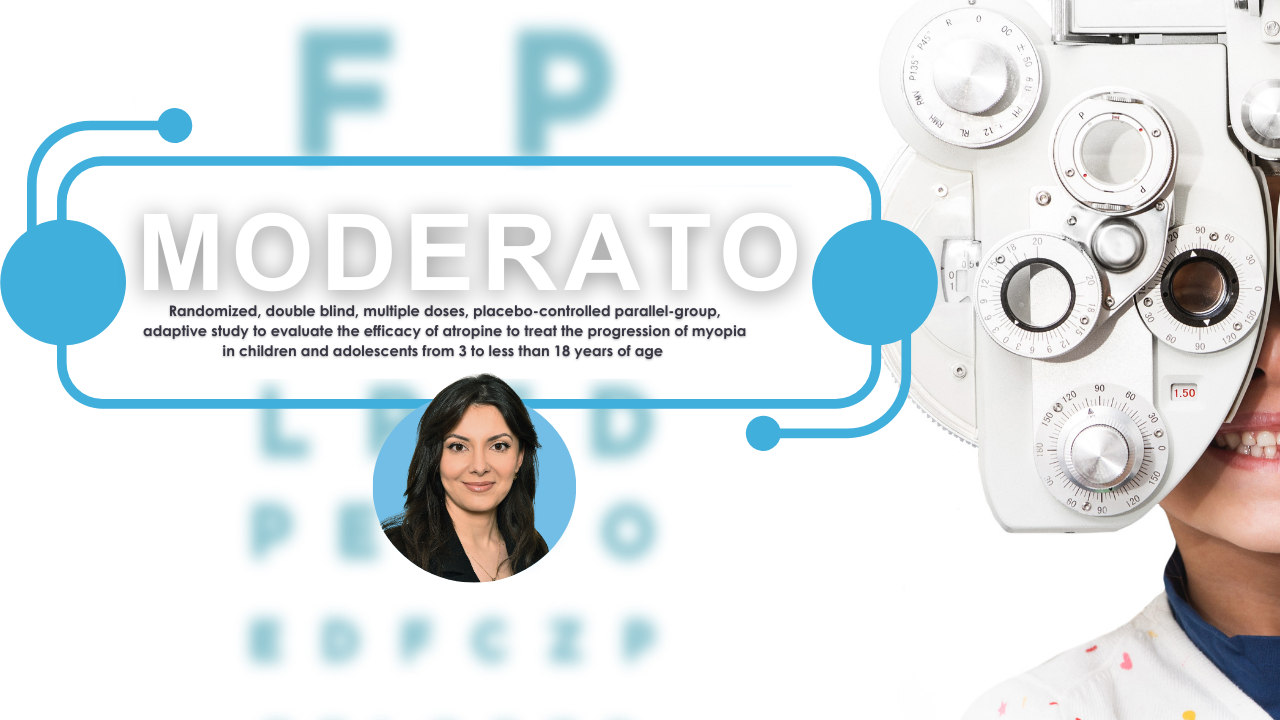
New Clinical Trial Seeks to Slow Progression of Nearsightedness in Children
A new international clinical trial is underway across Europe to test whether low-dose atropine eye drops can effectively slow the progression of [...]
13 Organizations Across Europe Join Forces for Novel ATMP on Faecal incontinence.
CVBF provides regulatory, site management, and monitoring support for the AMELIE (Anchored Muscle cELls for IncontinencE) project, Ana Dilo as our Clinical [...]
CVBF Head of Bio-Statistics lecturer at the Università Campus Bio-Medico di Roma
On 29 April 2023, Giorgio Reggiardo, our experienced research methodologist and biostatistician, held a presentation to the students of [...]
Clinical Research on Medical Devices. How to maximize the benefit for the patients? – CVBF will tackle the topic of Regulatory Aspects on Medical Devices
Donato Bonifazi, our CEO, will attend as a speaker, the conference "La ricerca clinica profit e no profit su farmaci [...]
CVBF supports the Rare Diseases Day 2023
This 28 February is Rare Diseases Day (RDD), an opportunity to celebrate people living with rare diseases and to raise [...]
Newsletter, FEBRUARY 2023
22nd February 2023 Newsletter February 2023 Clinical research is undergoing a moment of great change due to the increasingly use [...]
6th European Conference on Clinical Research
The 6th European Conference on Clinical Research organized by the European CRO Federation (EUCROF) took place on 07 & 08 February 2022 in Madrid (Spain) [...]
Pharmaceutics special issue dedicated to EPTRI
The Pharmaceutics journal dedicated a special issue, the “Scientific Highlights in the First European Paediatric Translational Research Infrastructure (EPTRI) Open Meeting” to highlight its important [...]
2nd International Conference on Rare Diseases, Tuesday 1st and Wednesday 2nd of March 2022, online
“The Balancing Act between Equity and Sustainability”. This year’s conference recognizes the need to promote and protect the human rights of all persons, including the [...]
ICH-CGP training course
CVBF is pleased to present its e-learning “ICH-Good Clinical Practice (GCP) Training Course”, aimed at providing a guide for all professionals involved in clinical research [...]
2022 starts with three new scientific publications
CVBF is excited to announce that three new research articles counting with the participation of experts from our consortium, were recently published. “Overview of the [...]
CVBF is now a member of the European Open Science Cloud
We are happy to announce that CVBF is now a member of the (EOSC). This will ensure compliance with new Open Science policy in the [...]